Brake Fluid White Paper on Copper
The Use of Dissolved Copper to
Indicate the Age of Brake Fluid
Dean R. Wheeler
Ph.D. in Chemical Engineering,
University of California, Berkeley
March 23, 2006
Introduction
This report, prepared for Phoenix FASCAR, is an
analysis of the chemical changes that take place as
brake fluid is used in service. The report addresses
how the amount of dissolved copper in the fluid can
serve as an indicator of the age and protective
ability of the fluid. The conclusions here come from
my interpretation of experimental data made
available to me, and my professional scientific and
engineering analysis. The references section at the
end gives some of the information sources I used in
preparing this report.
Brake fluid basics
Brake fluid is a hydraulic fluid mixture that
must function under many months of service and under
periodic high-temperature conditions. The main
governmental standard imposed on brake fluid is that
it have a high boiling point so that pockets of
vapor will not form in the braking system under
severe braking conditions. For instance,
moisture-free DOT 3 fluids must have a boiling point
above 400 °F. This can be compared to the boiling
point of 387 °F for pure ethylene glycol (automotive
antifreeze). In fact, many of the molecules that
make up DOT 3 and DOT 4 brake fluids can be
considered “larger chemical cousins” to ethylene
glycol. DOT 3 and DOT 4 brake fluids are
hygroscopic, meaning they will mix with and absorb
water, which lowers the boiling point just like with
antifreeze. This has led many people to incorrectly
believe that a low boiling point caused by water
absorption is the only thing that can go wrong with
brake fluid.
Current automotive brake systems contain steel
components, such as cylinders and valves, connected
by lengths of copper-alloy-lined steel tubing. Both
the steel and the copper components are unavoidably
subject to corrosion. One need hardly mention that
corrosion and wear of the metal surfaces can
interfere with the proper operation of these
components, leading to a diminished margin of
safety. Fortunately, the addition of standard
corrosion inhibitors by brake-fluid manufacturers
significantly slows the corrosion of critical steel
components, leading to much improved service life.
Recently there has been increased attention to
the fact that the protection offered by the
corrosion inhibitors in brake fluid does not last
indefinitely. As brake fluid ages in service, its
chemical constituents undergo a number of changes.
Ordinarily none of these fluid chemical changes are
immediately catastrophic, but cumulatively and over
time they lead to decreased braking-system
protection and performance. As already mentioned,
decreased boiling point (associated with water
absorption) is well recognized as a sign of
brake-fluid aging. However, this is not the complete
picture. As discussed below, an increased level of
dissolved copper in the solution is an important and
reproducible indicator that the brake fluid is no
longer effectively protecting metal surfaces from
corrosion.
The chemistry of corrosion
In order to better understand the changes taking
place in the brake fluid, it is necessary to have a
little background in corrosion science. The main
principle is that rust is a more natural and stable
state of iron than is a shiny machined steel part.
Rust is composed of iron mixed with oxygen.
Similarly, other metals such as copper corrode
spontaneously by reacting with oxygen. In practical
environments it is impossible to fully prevent
corrosion; instead it is a matter of trying to slow
it down as much as possible.
For most metals (gold is a notable exception)
exposed to dry air, a thin layer of the metal on the
surface reacts with oxygen in the air to form a
dense oxide film. This film "passivates" and
protects the rest of the metal by acting as a
barrier to greatly reduce further reaction with
oxygen. Unfortunately, when water or a similar
solvent contacts the metal, it partially dissolves
the protective metal oxide skin, leading to
increased corrosion in the presence of oxygen. The
problem is typically made even worse in situations
where there are aggressive chemicals or high
temperatures present. Note that most of what we know
about metals and corrosion is for the case of water
mixtures; however, the same principles apply to
brake fluid.
A simplified corrosion reaction for a metal in
liquid looks something like this:
metal + dissolved oxygen + acid =
dissolved metal.
There are a few ways to "starve" this reaction
and therefore slow down the corrosion: First, one
can attempt to reduce the amount of dissolved oxygen
in the solution. In the case of brake systems, it is
nearly impossible to prevent oxygen from absorbing
into the solution due to the fluid-air interface in
the master cylinder, and the slow leakage of oxygen
into the system through rubber parts. A second
scheme is to reduce the acid in the system by adding
chemicals that are alkaline. This scheme is used in
brake fluids. A third scheme is to add chemicals to
the system that stick to and coat the metal surface,
providing a barrier in addition to the metal oxide
film to slow things down. This scheme is also used
in brake fluids.
Water is known to degrade the integrity of the
oxide film on metals; however, water is not the only
solvent that can do this. Corrosion can take place
in other liquids, such as those that make up brake
fluid. Furthermore, there is no practical way to
keep brake fluid completely moisture free, so there
will always be some water present near the metal
surface. I am aware of only two scientific studies
of corrosion in brake systems (both are listed in
the references section). Neither showed that the
amount of absorbed water in a brake fluid was a main
controlling factor in how fast the metals corroded.
Corrosion with different metals
There is one more complication I need to
introduce into the corrosion picture, namely that
individual metals differ in their susceptibility to
corrosion and also can corrode one another. This can
work to advantage or disadvantage depending on the
system. Here I consider three metals: zinc, iron,
and copper. Zinc is the least "noble" of the three -
meaning most susceptible to corrosion - while copper
is the most noble. I give some examples of this
metal-to-metal corrosion behavior below.
Galvanized nails used in home construction are
steel nails that have been dipped in molten zinc to
form a zinc coating on the outside. Because zinc is
less noble than iron, it will corrode before iron
will. If the zinc coating is ever broken, and
dissolved oxygen gets to the exposed steel surface,
the surrounding zinc will "sacrifice" itself and
react with the oxygen before the iron does, and thus
protect the iron.
Copper, being the most noble of the three metals
I listed, is the best protected against oxidation or
corrosion under normal exposure to dissolved oxygen.
This is the reason that plumbing pipes in homes are
generally made of copper, not steel. However, in a
situation where copper metal has already been
corroded and dissolved into a liquid, it will attack
any iron metal (steel) it comes in contact with.
This is because, like zinc does for iron, the iron
will sacrifice itself for the copper. The result is
that dissolved copper will come out of solution and
plate onto the surrounding steel, while a
proportional amount of iron will dissolve and go
into solution. While the initial corrosion reaction
of copper requires oxygen and acid, the second
reaction where dissolved copper corrodes the iron
does not have this requirement. This chemistry is
important in explaining what can happen in brake
systems with aged and degraded brake fluid.
The Highway Traffic Safety Administration of the
U.S. government conducted a six-year engineering
analysis (EA94-0038), culminating in a report in
year 2000, to investigate decreased performance and
possible failure of anti-lock braking systems on
light trucks and SUVs. During the course of the
investigation the agency contracted the services of
the National Institute for Science and Technology
(NIST). The scientific tests by NIST indicated that
it was possible for corrosion to take place in the
brake system so as to form deposits of foreign
copper particles around the sealing surfaces of the
steel PWM valve. The effects of a leaking PWM valve
on vehicle braking performance were studied in a
separate report (EA95-026). The important lesson, as
I discuss below, is that copper is not necessarily
benign and inert in the presence of iron and could
lead to degraded braking performance.
The role of corrosion inhibitors in brake
fluid
Corrosion inhibitors come in many varieties, but
the ones used in brake fluid are typically based on
a chemical group called "amine." The amine-based
inhibitors are well known as being able to protect
iron or steel from corrosion in aggressive
high-temperature liquid environments. For instance,
amines are widely used as corrosion inhibitors in
steam boilers. Individual amine inhibitors work in
one of two different ways: (1) by reducing the acid
level (neutralizing or buffering amines) and (2) by
forming a water-repelling barrier film on the metal
surface (filming amines).
In brake fluid, the amount of amines present is
usually reported in terms of "reserve alkalinity," a
scientific term that indicates how much acid can be
added to the brake fluid before the neutralizing
ability of the amines is exhausted. However,
neutralizing amines alone will not
adequately prevent corrosion in the presence of
dissolved oxygen. This is because even in a buffered
alkaline solution (high pH) there is still a small
amount of acid present to slowly feed the corrosion
reaction. To give full protection, the inhibitor
package requires the help of the filming amines as
well. However, reserve alkalinity does not
necessarily account for the presence or absence of
the filming amines, and so gives only a partial
picture of how much protection is left in a given
sample of brake fluid.
A fact that is rarely appreciated is that the
amines do not protect copper as well as
they protect iron. This is backed up by the
observation that dissolved-copper levels in brake
fluid begin rising almost immediately upon the fluid
being put into service, and the levels rise
consistently throughout service. On the other hand,
dissolved-iron levels do not begin to rise
noticeably until the corrosion inhibitors have
already been significantly depleted.
A significant experimental study was conducted
jointly by researchers at Delphi, Union Carbide, and
General Motors and published by the Society of
Automotive Engineers in 1997 (see references
section). The researchers examined the durability of
corrosion protection in brake fluids. They found
that the corrosion protection declined sharply with
time in service. The following numbers are telling:
Reserve alkalinity was between 10 and 20% of its
initial level for the tested fluids after 30 months
of service (about 23,000 miles). Furthermore, they
found that by 40 months of service (about 34,000
miles) most of the amine inhibitors were deactivated
by thermal reactions that turned them into
non-inhibiting chemicals. Interestingly, they found
that around 60% of the amines-both active and
inactive-were lost entirely from the brake fluid by
this time. They believed this to be due to the
amines being volatilized (evaporated) into the air
space of the master cylinder and by permeating out
through rubber components.
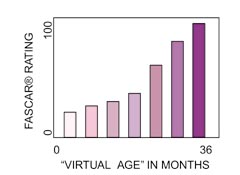
The role of dissolved copper in brake
fluid
Experiments by both Phoenix Systems and the
industry researchers mentioned above have found that
dissolved copper levels in brake fluid increase
nearly constantly with time of service. The SAE
paper reports copper levels at 150 to 300 ppm (parts
per million) after 30 months of service. In
contrast, the respective levels of dissolved iron
and zinc are significantly smaller and do not follow
as clear of a trend with time. It is true that
dissolved iron could be used as an indicator of a
problem, because elevated levels of dissolved iron
clearly show that corrosion has occurred. However,
this may not be the best practice in a routine
maintenance program that is intended to keep
corrosion low at all times, rather than respond to a
problem after it develops. In summary, copper
concentration level in the fluid is one of the
clearest available indicators of time-in-service for
brake fluid. It can serve like wear indicators on
brake pads do, warning when a problem is imminent
rather than just warning when a problem has already
developed.
Moreover, copper is much more than a benign
indicator of brake-fluid service time. Copper plays
a key role in the chemistry of corrosion for the
brake system. The problem as discussed above is that
relatively unprotected and large copper surfaces can
corrode almost from the outset of fluid service. The
corrosion of the copper-lined tubing is less
worrisome than it is for the moving steel parts in
the brake system, because close tolerances are not
as essential for the tubing. The problem, however,
is that the dissolved copper then goes on to attack
and deposit itself on the steel surfaces once the
corrosion inhibitors are sufficiently depleted. The
presence of high levels of dissolved copper in the
brake fluid indicates that the steel surfaces in the
brake system are already or will soon be under
attack.
The SAE study included an attempt to create
artificially aged braking fluid for testing
purposes. The researchers found that two things were
required to create fluid that behaved similarly to
fluid that had seen many months of vehicle service:
(1) significant amounts of added copper and (2)
elevated temperatures in order to thermally degrade
the corrosion inhibitors. Simple thermal degradation
without adding copper did not lead to fluid
that correctly mimicked the corrosive action of
truly old brake fluid. In fact, the researchers
speculated that the copper metal added to the system
acts as a catalyst to promote the degradation of the
amine-based inhibitors.
My analysis suggests that the presence in the
brake system of copper as well as amine-based
corrosion inhibitors is an unfortunate combination
that in the end works to promote iron corrosion. It
is known that amines associate strongly with
dissolved copper. Any filming amines that associate
with copper in solution cannot at the same time do
their job of protecting iron. Therefore, elevated
levels of dissolved copper may interfere with the
effectiveness of the filming amines in preventing
corrosion of the steel surfaces.
Additional factors in fluid age
There are additional factors that can aggravate
the corrosion problems mentioned above. For
instance, anti-lock braking systems create greater
circulation of brake fluid in the system. This
circulation causes dissolved oxygen and dissolved
copper to transport more freely throughout the
system, likely leading to greater corrosion exposure
that inhibitors must then counteract. This could
lead to more rapid depletion of inhibitors than in a
non-ABS system.
Similarly, city driving with its more extensive
use of braking will lead to elevated temperatures in
the system. Spontaneous chemical reactions always
speed up at higher temperatures. Therefore, higher
temperatures accelerate all of the undesirable
corrosion-both of copper and iron-as well as the
processes that degrade the inhibitor package.
Therefore, an automobile that has seen "hard
driving" with frequent use of brakes is likely to
show greater depletion of the inhibitors and loss of
corrosion protection, as well as greater copper
concentration, for a given time or mileage in
service. So the use of copper concentration as an
indicator will naturally account for some degree of
variation in user abuse of the braking system. On
the other hand, elevated temperatures will tend to
reduce the amount of water that would
otherwise be in the brake fluid. This is because
water, with its lower boiling point, will volatilize
more strongly than other components as temperature
is increased.
Other measurements of fluid age
In contrast to dissolved-copper measurements,
boiling-point and reserve-alkalinity measurements
are less effective as indicators of fluid-service
time. This is because these two quantities can vary
so widely from one manufacturer's brake-fluid
formulation to the next. Unlike in a laboratory
experiment, a technician in the field has no
foolproof way of knowing the baseline level of
either quantity. For relatively new cars it is
reasonable to assume that they contain the
OEM-formulated brake fluid, but for a car that has
been in service for a few years the brake fluid is a
big question mark and could by that point even be a
mixture of different manufacturers' brake fluids.
For instance, the SAE paper notes the wide
variations, with reserve alkalinity levels for fresh
commercial brake fluids ranging from a low of 3 to a
high of 120. These researchers also warn that
reserve alkalinity only measures general
acid-buffering ability and not the concentration of
particular inhibitors. Therefore, it would be
unlikely that one could reliably predict either (1)
months of service of the brake fluid or (2)
remaining strength of the full corrosion-inhibitor
package using boiling point or reserve alkalinity.
References
G.L. Jackson, P. Levesque, and F.T. Wagner,
"Improved Methods for Testing the Durability of
Corrosion Protection in Brake Fluids," Paper 971007,
SAE Technical Paper Series (1997).
R.E. Ricker, J.L. Fink, A.J. Shapiro, L.C. Smith,
and R.J. Schaefer, "Preliminary Investigations Into
Corrosion in Anti-Lock Braking Systems," Internal
Report 6233, National Institute of Standards and
Technology, U.S. Dept. of Commerce (1998). |

![]()